Introduction to Peptides: Understanding the Basics of These Powerful Molecules
By Licensed Peptides | August 20th, 2025
Disclaimer: All articles and product details provided on this website are intended for educational and informational purposes only. The products listed here are for in-vitro research only. In-vitro studies are conducted outside of living organisms. These products are not intended as medicines or drugs and have not been approved by the FDA to prevent, treat, or cure any medical condition, ailment, or disease. The direct or indirect administration of these substances to humans or animals is unequivocally prohibited under applicable law.
What Are Peptides?
 Peptides are molecules of two or more amino acids linked by peptide bonds—covalent links formed when the carboxyl group of one amino acid reacts with the amine group of another in a condensation reaction, releasing water. The resultant CO–NH bond is also called an amide bond. The term “peptide” comes from the Greek πέσσειν, meaning “to digest.” Peptides are fundamental in biochemistry, widely present in living organisms, and continually synthesized in labs to explore new therapeutic possibilities.
Peptides are molecules of two or more amino acids linked by peptide bonds—covalent links formed when the carboxyl group of one amino acid reacts with the amine group of another in a condensation reaction, releasing water. The resultant CO–NH bond is also called an amide bond. The term “peptide” comes from the Greek πέσσειν, meaning “to digest.” Peptides are fundamental in biochemistry, widely present in living organisms, and continually synthesized in labs to explore new therapeutic possibilities.
How Are Peptides Formed?
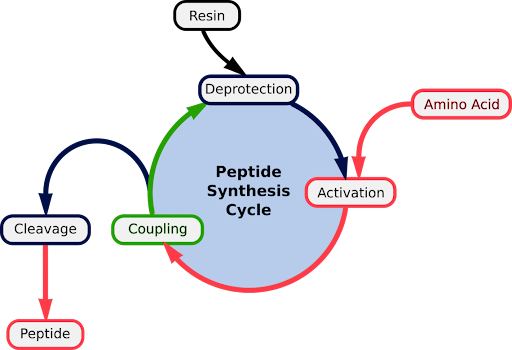
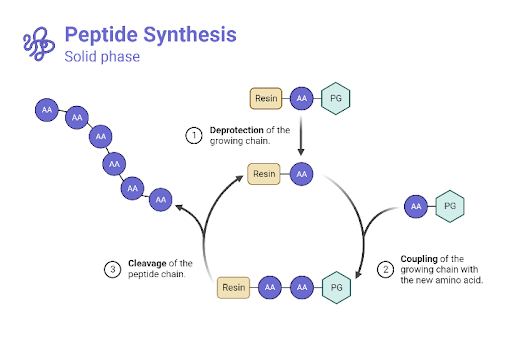 Peptides are produced both naturally—for example, ribosomal and nonribosomal peptides—and synthetically using methods like liquid-phase or solid-phase peptide synthesis (SPPS). SPPS is the standard technique, enabling precise, repetitive peptide assembly.
Peptides are produced both naturally—for example, ribosomal and nonribosomal peptides—and synthetically using methods like liquid-phase or solid-phase peptide synthesis (SPPS). SPPS is the standard technique, enabling precise, repetitive peptide assembly.
Historical milestones:
- 1901 – Emil Fischer & Ernest Fourneau synthesized the first artificial peptide.
- 1953 – Vincent du Vigneaud created oxytocin, the first polypeptide.
Peptide Terminology
- Dipeptide – two amino acids
- Tripeptide – three amino acids
- Oligopeptide – fewer than 10 amino acids
- Polypeptide – typically 10 or more amino acids
- Proteins – usually over 40–50 amino acids, though distinctions can blur (e.g., amyloid beta is long but a peptide; insulin is relatively small but considered a protein).
Types of Peptides
- Ribosomal peptides – synthesized via mRNA translation; often act as hormones or signaling molecules. Examples include tachykinins and opioid peptides.
- Nonribosomal peptides – assembled by specialized enzymes, often forming cyclic structures (e.g., glutathione).
- Milk peptides – derived from milk proteins via digestion or fermentation.
- Peptones – peptides from animal proteins, frequently used as lab nutrients for microbial cultures.
- Peptide fragments – shorter sequences occurring through natural breakdown or lab-generated processes.
Key Concepts in Peptide Science
 Amino Acids: The basic units forming peptides, each with both amine and carboxyl functional groups.
Amino Acids: The basic units forming peptides, each with both amine and carboxyl functional groups.- Cyclic Peptides: Peptides structured in ring form, such as melanotan-2 or Bremelanotide (PT-141).
- Peptide Sequence: The specific order of amino acids in the chain.
- Peptide Bond: The covalent linkage formed via condensation between amino acids.
- Peptide Mapping: A technique to break down and analyze peptide or protein sequences.
- Peptide Mimetics: Molecules designed to mimic the biological functions of natural peptides.
- Peptide Fingerprint: Unique chromatographic fragmentation patterns used to identify peptides.
- Peptide Library: A curated collection of peptides, often synthesized via SPPS, used in biochemical research and drug discovery.
Stay Ahead of the Curve
Get exclusive updates on events, promotions, and special offers.
