Understanding Peptide Bonds: The Chemistry That Connects Amino Acids
By Licensed Peptides | August 20th, 2025
Disclaimer: All articles and product details provided on this website are intended for educational and informational purposes only. The products listed here are for in-vitro research only. In-vitro studies are conducted outside of living organisms. These products are not intended as medicines or drugs and have not been approved by the FDA to prevent, treat, or cure any medical condition, ailment, or disease. The direct or indirect administration of these substances to humans or animals is unequivocally prohibited under applicable law.
Understanding the Peptide Bond
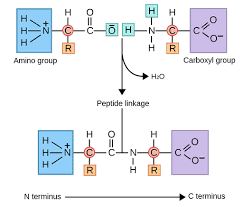 A peptide bond is a type of covalent bond that links two amino acids together. This bond forms when the carboxyl group of one amino acid chemically reacts with the amino group of another, releasing a molecule of water in a condensation reaction. The bond formed between the carbon and nitrogen atoms—specifically a C–N (amide) linkage—is what’s referred to as a peptide bond, and the resulting molecule is categorized as an amide.
A peptide bond is a type of covalent bond that links two amino acids together. This bond forms when the carboxyl group of one amino acid chemically reacts with the amino group of another, releasing a molecule of water in a condensation reaction. The bond formed between the carbon and nitrogen atoms—specifically a C–N (amide) linkage—is what’s referred to as a peptide bond, and the resulting molecule is categorized as an amide.
How a Peptide Bond Is Formed
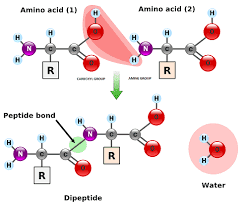 To successfully create a peptide bond, the participating amino acids must be aligned in a way that allows the acid group (–COOH) of one to react with the amine group (–NH₂) of the other. This is most easily visualized in the formation of a dipeptide, which is composed of just two amino acids linked by a single peptide bond.
To successfully create a peptide bond, the participating amino acids must be aligned in a way that allows the acid group (–COOH) of one to react with the amine group (–NH₂) of the other. This is most easily visualized in the formation of a dipeptide, which is composed of just two amino acids linked by a single peptide bond.
However, this process isn’t limited to just two amino acids. Many amino acids can be sequentially linked to form longer chains:
- Chains containing fewer than 50 amino acids are typically called peptides
- Chains with 50 to 100 amino acids are considered polypeptides
- Sequences with more than 100 amino acids are generally referred to as proteins
If you’d like a deeper breakdown of these classifications, licensed peptides provides a helpful comparison on their Peptides vs. Proteins glossary page.
Peptide Bond Breakdown: Hydrolysis
Peptide bonds can be broken through a chemical process called hydrolysis, where a water molecule reacts with the bond. While this breakdown happens slowly under normal conditions, peptide bonds are still considered metastable—meaning they can be disrupted over time in the presence of water.
Each peptide bond hydrolyzed releases approximately 10 kJ/mol of free energy. Additionally, peptide bonds have a characteristic absorbance range between 190–230 nm, which is useful in analytical methods such as spectroscopy.
In living systems, specialized enzymes facilitate both the formation and hydrolysis of peptide bonds. Many biologically active compounds—including hormones, antibiotics, neurotransmitters, and anticancer agents—are built from peptides, and are often referred to as proteins when the sequence length warrants it.
Structural Characteristics of the Peptide Bond
Extensive structural studies, including X-ray crystallography, have provided insight into the physical properties of peptide bonds. Findings indicate that peptide bonds are planar and rigid in structure. This rigidity comes from resonance stabilization within the amide group, where the lone pair of electrons on the nitrogen is delocalized toward the carbonyl oxygen, resulting in a partial double bond character.
This resonance affects bond lengths:
- The N–C (peptide) bond is shorter than the N–Cα bond
- The C=O bond is longer than in typical carbonyl compounds
In most peptides, the carbonyl oxygen and amide hydrogen adopt a trans configuration, which is favored over the cis form due to reduced steric hindrance and lower overall energy.
Polarity and Electron Distribution in Peptide Bonds
Although single bonds generally allow for free rotation, the partial double-bond nature of the peptide bond restricts rotational movement. The resonance between the lone pair on nitrogen and the carbonyl group creates a significant stabilization, making the peptide bond about 40% double bond in character, and therefore quite rigid.
This electron delocalization also introduces permanent polarity in the peptide bond:
- The oxygen carries a partial negative charge (approximately -0.28)
- The nitrogen carries a partial positive charge (approximately +0.28)
This dipole plays a role in how peptides and proteins fold, interact, and maintain their structure in biological environments.
This overview of peptide bonds offers insight into their chemical formation, structural properties, and behavior under different conditions. Whether for theoretical understanding or applied research, recognizing the nature of the peptide bond is fundamental in fields like biochemistry, molecular biology, and peptide-based therapeutics.
Stay Ahead of the Curve
Get exclusive updates on events, promotions, and special offers.
