SLUPP332 Capsules
$279.99
SLU-PP-332 is an investigational compound under study for its ability to reproduce some of the physiological effects typically associated with exercise. Its mechanism of action involves activation of estrogen-related receptors (ERRα, ERRβ, and ERRγ), which are central regulators of energy metabolism. Through this pathway, the compound has been shown to enhance mitochondrial efficiency, increase fatty acid oxidation, and elevate overall energy expenditure.
In preclinical studies involving both diet-induced and genetically obese mice, administration of SLU-PP-332 produced significant metabolic improvements. Animals receiving the compound demonstrated reduced fat mass and greater rates of fatty acid utilization, while food intake and physical activity remained unchanged. These findings suggest that the compound alters systemic energy balance, creating a more favorable metabolic profile without requiring lifestyle modification.
Additional investigations have highlighted the compound’s effects on skeletal muscle physiology. Treatment was associated with a higher proportion of oxidative muscle fibers, a shift that supports endurance capacity. Functional outcomes reflected this change, as mice receiving SLU-PP-332 exhibited prolonged exercise tolerance. Mechanistic studies attributed these effects to enhanced mitochondrial respiration and improved cellular bioenergetics within muscle tissue.
$279.99
$279.99
Peptide Capsules
Purchase Peptides
Purchase Blends
Overview
SLU-PP-332 has also been evaluated in cardiac disease models. In mouse models of heart failure, the compound improved myocardial function, preserved mitochondrial ultrastructure, and promoted upregulation of oxidative phosphorylation and lipid metabolism pathways. These data indicate potential therapeutic relevance for conditions characterized by impaired cardiac energetics.
While the results across obesity, skeletal muscle, and heart failure models are encouraging, SLU-PP-332 remains in the preclinical stage of development. Human studies will be required to establish its safety, efficacy, and therapeutic utility. At present, its promise lies in early evidence that pharmacologic activation of ERR pathways may serve as a novel strategy for treating metabolic and cardiovascular disease.
It is well established that consistent physical activity contributes to improved health by reducing the risk of cardiovascular disease, limiting obesity, enhancing mood, supporting cognitive function, and helping to prevent or manage a range of chronic conditions. Despite these benefits, efforts to reproduce the physiological effects of exercise with pharmacological agents have often been unsuccessful.
A new line of investigation has introduced SLU-PP-332, an experimental compound designed to mimic some of the metabolic outcomes of exercise. SLU-PP-332 acts as an agonist of estrogen-related receptors, particularly within the alpha and gamma subclasses, which play a critical role in regulating energy metabolism.
Preclinical studies have demonstrated multiple beneficial effects. The compound has been shown to enhance skeletal muscle endurance, facilitate weight reduction, improve markers of cardiovascular function, and provide potential protection to the nervous system against age-associated decline.
Because SLU-PP-332 represents the closest attempt to date at pharmacologically replicating the systemic benefits of exercise, it has attracted significant attention within the research community. Although still at an early stage of development, its promise has stimulated interest in its possible applications across metabolic and age-related diseases.
SLU-PP-32: Structure
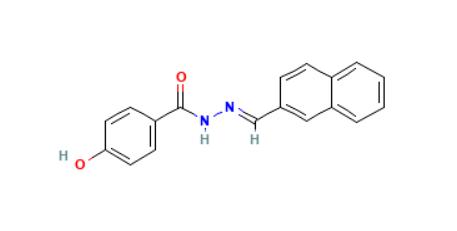
Chemical Formula: C₁₈H₁₄N₂O₂
Molecular Weight: 290.3 g/mol
PubChem CID: 5338394
CAS No.: 303760-60-3
Synonyms: 4-Hydroxy-N′-(naphthalen-2-ylmethylene) benzohydrazide
Source: PubChem
SLU-PP-32: Research
SLU-PP-332: Considerations on Bioavailability
A key feature that distinguishes SLU-PP-332 is its ability to effectively reach estrogen-related receptors (ERRs) throughout the body after administration. This property enables its use in in vivo studies, where activity within living systems is essential. Historically, many ERR agonists were limited to in vitro applications, as they were either metabolically unstable or unable to access their intended cellular targets.
SLU-PP-332 represents one of the first compounds with demonstrated ERR-binding capacity in living cells, along with a safety profile suitable for further evaluation. Importantly, it is also among the earliest ERRα agonists to be successfully developed. While agonists targeting ERRβ and ERRγ have been easier to design, ERRα agonists have long remained a challenge. Prior to SLU-PP-332, no compound was available that could selectively activate ERRα, despite ERRα being the first member of the receptor family to be identified.
This breakthrough has positioned SLU-PP-332 as a pivotal compound in the advancement of exercise mimetic research, sparking considerable interest in its potential applications across metabolic and physiological fields.
SLU-PP-332: A Pharmacological Approach to Exercise Mimicry
The benefits of physical activity are broad, encompassing improvements in cardiovascular function, body composition, bone strength, metabolic health, and cognitive performance. Exercise is also linked to delayed progression of age-related decline. Despite these well-established effects, consistent participation in exercise remains challenging for many due to personal, physical, or logistical barriers. This has driven interest in compounds capable of reproducing the physiological benefits of exercise through pharmacological means, sometimes referred to as “exercise mimetics.
Several drug classes have demonstrated partial overlap with the effects of exercise. For example, GLP-1 receptor agonists, such as semaglutide, are known to promote weight reduction and appetite control. Growth hormone–releasing hormone (GHRH) analogs like sermorelin and CJC-1295 have been associated with increased lean body mass, decreased adiposity, and improved cardiovascular outcomes. In addition, agents such as Selank and Semax, which act on neurotrophic pathways, have shown the ability to enhance cognitive function and counteract aspects of aging.
These examples, however, represent only incremental advances. Few compounds have demonstrated the capacity to influence cellular respiration, a process central to energy metabolism. Cellular respiration occurs primarily within mitochondria, the organelles responsible for energy production. Exercise enhances mitochondrial function and biogenesis, leading to improved glucose handling, increased metabolic rate, reduced insulin resistance, better endurance, and vascular health benefits.
SLU-PP-332 has emerged as a compound capable of directly modulating mitochondrial function. Preclinical studies indicate that it enhances endurance capacity, facilitates weight reduction, and improves energy metabolism without altering food consumption or requiring increased physical activity. In one study, obese mice receiving SLU-PP-332 twice daily over a four-week period lost approximately 12 percent of their body weight. Beyond its role in metabolism, the compound has also shown potential in ameliorating features of metabolic syndrome, a condition that has proven difficult to treat with existing therapies.
SLU-PP-332 and Skeletal Muscle Adaptation
Estrogen-related receptors (ERRs) in skeletal muscle are activated in response to physical stress, such as that induced by exercise. Much like muscle fibers adapt and grow stronger under training conditions, ERR expression rises when oxygen demand increases within muscle tissue. Exercise-induced oxygen deficits trigger signaling pathways that stimulate the production of additional ERRs, thereby enhancing muscle capacity.
SLU-PP-332 has demonstrated a similar effect, activating ERR pathways in a way that supports the efficient delivery of oxygen and nutrients to muscle cells. This enhanced metabolic support contributes to improved muscular endurance and overall functional performance, paralleling the adaptive benefits typically observed with regular exercise.
SLU-PP-332 and Cardiovascular Support
As a broad-spectrum ERR agonist, SLU-PP-332 exerts activity across multiple receptor subtypes. Preclinical investigations in mouse models of cardiac failure have shown promising results. Treatment was associated with improvements in left ventricular ejection fraction, reduced cardiac fibrosis, and increased survival in models of pressure overload–induced heart failure. Additionally, the compound supported normalization of fatty acid oxidation within cardiac tissue, thereby promoting energy balance in the failing heart.
Fibrosis remains one of the most serious challenges in heart failure management, as the replacement of healthy myocardium with non-functional scar tissue disrupts contractility and can lead to arrhythmias or impaired conduction. The ability to limit fibrotic progression is a critical component of preserving cardiac function and delaying the progression toward terminal failure.
One proposed mechanism by which SLU-PP-332 exerts its cardioprotective effects is through the regulation of autophagy. Autophagy serves as a cellular recycling process, clearing damaged components to allow replacement with new, functional material. ERR activation has been linked to regulation of autophagy via transcription factor EB (TFEB). By stimulating TFEB activity, ERRs may promote clearance of dysfunctional material within cardiomyocytes and help reduce the accumulation of scar tissue, ultimately supporting the maintenance of viable cardiac muscle.
SLU-PP-332: Links to Caloric Restriction and Healthy Aging
Renal function naturally declines with age, but severe deterioration is often driven by conditions such as hypertension and metabolic disease. These disorders accelerate inflammation and mitochondrial dysfunction in the kidneys, ultimately contributing to chronic kidney disease. Because of this, the kidneys are frequently studied as a marker of systemic aging and provide a valuable model for investigating interventions that may slow age-related decline.
Studies in both human and murine models have shown that estrogen-related receptor (ERR) expression decreases with advancing age, except in individuals subjected to long-term caloric restriction. Caloric restriction is one of the most extensively validated interventions for extending lifespan and healthspan, consistently demonstrating protective effects against age-associated decline. In animal studies, calorie-restricted mice displayed improved renal function when compared with age-matched controls.
Building on these findings, researchers have suggested that ERR signaling plays an essential role in long-term health maintenance. Caloric restriction has been observed to protect against age-related increases in urinary albumin, elevations in inflammatory cytokines, and progressive mitochondrial dysfunction. Interestingly, many of these benefits are also reproduced in mice treated with SLU-PP-332, even in the absence of dietary restriction. Evidence indicates that ERRα activation is particularly effective in mimicking the protective outcomes of caloric restriction, and SLU-PP-332 remains the only available ERRα agonist with demonstrated activity in in vivo models.
Mitochondrial dysfunction is widely regarded as a hallmark of aging, closely tied to impaired cellular homeostasis. As mitochondrial efficiency declines, oxygen free radicals accumulate, leading to oxidative stress and structural damage. This process contributes to cancer, neurodegeneration, and other age-related conditions. Enhancing mitochondrial resilience is therefore a major focus in anti-aging research. SLU-PP-332 has shown promise in this area by promoting mitochondrial integrity, reducing free radical damage, and supporting cellular function, thereby helping to delay the onset of age-related disease processes.
Referenced Citations
Loss of skeletal muscle estrogen-related receptors leads to severe exercise intolerance
The Estrogen Receptor-Related Orphan Receptors Regulate Autophagy through TFEB
Losby, McKenna et al.
Molecular Pharmacology, Volume 106, Issue 4, 164 – 172
https://pubs.acs.org/doi/10.1021/acschembio.2c00720
Mimicking exercise: what matters most and where to next?
John A. Hawley, Michael J. Joyner, Daniel J. Green
ALL ARTICLES AND PRODUCT INFORMATION PROVIDED ON THIS WEBSITE ARE FOR INFORMATIONAL AND EDUCATIONAL PURPOSES ONLY.
The products available on this website are intended solely for in-vitro research purposes (Latin: “in glass”), meaning they are used in experiments conducted outside a living organism. These products are not medicines or drugs, have not been evaluated or approved by the U.S. Food and Drug Administration (FDA), and are not intended to diagnose, treat, cure, or prevent any disease or medical condition. Any administration to humans or animals, whether by ingestion, injection, or other means, is strictly prohibited by law.
Test
Storage Instructions:
All of our products are manufactured using the Lyophilization (Freeze Drying) process, which ensures that our products remain 100% stable for shipping for up to 3-4 months.
Once the peptides are reconstituted (mixed with bacteriostatic water), they must be stored in the fridge to maintain stability. After reconstitution, the peptides will remain stable for up to 30 days.
Lyophilization is a unique dehydration process, also known as cryodesiccation, where the peptides are frozen and then subjected to low pressure. This causes the water in the peptide vial to sublimate directly from solid to gas, leaving behind a stable, crystalline white structure known as lyophilized peptide. The puffy white powder can be stored at room temperature until you’re ready to reconstitute it with bacteriostatic water.
Once peptides have been received, it is imperative that they are kept cold and away from light. If the peptides will be used immediately, or in the next several days, weeks or months, short-term refrigeration under 4C (39F) is generally acceptable. Lyophilized peptides are usually stable at room temperatures for several weeks or more, so if they will be utilized within weeks or months such storage is typically adequate.
However, for longer term storage (several months to years) it is more preferable to store peptides in a freezer at -80C (-112F). When storing peptides for months or even years, freezing is optimal in order to preserve the peptide’s stability.
For further information on proper storage techniques, click the link below:




