-

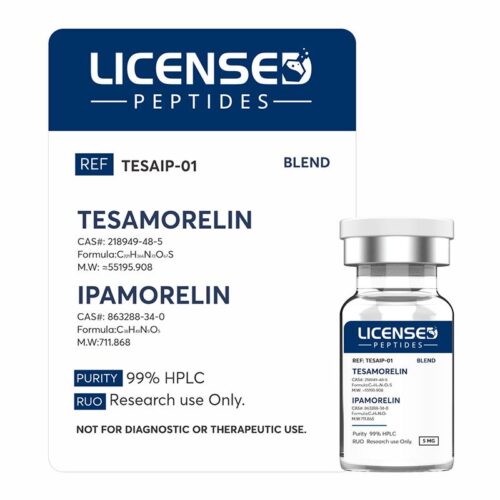
 Tesamorelin is a synthetic peptide analogue of growth hormone–releasing hormone (GHRH), composed of 44 amino acids with a structural modification that enhances its stability and half-life. Although it has gained regulatory approval as Egrifta® for the treatment of HIV-associated lipodystrophy, tesamorelin is also widely investigated in the broader research context.
Tesamorelin is a synthetic peptide analogue of growth hormone–releasing hormone (GHRH), composed of 44 amino acids with a structural modification that enhances its stability and half-life. Although it has gained regulatory approval as Egrifta® for the treatment of HIV-associated lipodystrophy, tesamorelin is also widely investigated in the broader research context. -
 TB-500 (Thymosin Beta-4) is a synthetic peptide consisting of 43 amino acids. Preclinical studies in animal models indicate that Thymosin Beta-4 supports angiogenesis, modulates wound healing, reduces inflammation, and mitigates oxidative stress in both cardiac and neurological tissues. It plays a critical role in cytoprotection, tissue repair, regeneration, and structural remodeling following injury. Due to these properties, it has also attracted significant interest within the field of anti-aging research.
TB-500 (Thymosin Beta-4) is a synthetic peptide consisting of 43 amino acids. Preclinical studies in animal models indicate that Thymosin Beta-4 supports angiogenesis, modulates wound healing, reduces inflammation, and mitigates oxidative stress in both cardiac and neurological tissues. It plays a critical role in cytoprotection, tissue repair, regeneration, and structural remodeling following injury. Due to these properties, it has also attracted significant interest within the field of anti-aging research. -

 SLU-PP-332 is an investigational compound under study for its ability to reproduce some of the physiological effects typically associated with exercise. Its mechanism of action involves activation of estrogen-related receptors (ERRα, ERRβ, and ERRγ), which are central regulators of energy metabolism. Through this pathway, the compound has been shown to enhance mitochondrial efficiency, increase fatty acid oxidation, and elevate overall energy expenditure. In preclinical studies involving both diet-induced and genetically obese mice, administration of SLU-PP-332 produced significant metabolic improvements. Animals receiving the compound demonstrated reduced fat mass and greater rates of fatty acid utilization, while food intake and physical activity remained unchanged. These findings suggest that the compound alters systemic energy balance, creating a more favorable metabolic profile without requiring lifestyle modification. Additional investigations have highlighted the compound’s effects on skeletal muscle physiology. Treatment was associated with a higher proportion of oxidative muscle fibers, a shift that supports endurance capacity. Functional outcomes reflected this change, as mice receiving SLU-PP-332 exhibited prolonged exercise tolerance. Mechanistic studies attributed these effects to enhanced mitochondrial respiration and improved cellular bioenergetics within muscle tissue.
SLU-PP-332 is an investigational compound under study for its ability to reproduce some of the physiological effects typically associated with exercise. Its mechanism of action involves activation of estrogen-related receptors (ERRα, ERRβ, and ERRγ), which are central regulators of energy metabolism. Through this pathway, the compound has been shown to enhance mitochondrial efficiency, increase fatty acid oxidation, and elevate overall energy expenditure. In preclinical studies involving both diet-induced and genetically obese mice, administration of SLU-PP-332 produced significant metabolic improvements. Animals receiving the compound demonstrated reduced fat mass and greater rates of fatty acid utilization, while food intake and physical activity remained unchanged. These findings suggest that the compound alters systemic energy balance, creating a more favorable metabolic profile without requiring lifestyle modification. Additional investigations have highlighted the compound’s effects on skeletal muscle physiology. Treatment was associated with a higher proportion of oxidative muscle fibers, a shift that supports endurance capacity. Functional outcomes reflected this change, as mice receiving SLU-PP-332 exhibited prolonged exercise tolerance. Mechanistic studies attributed these effects to enhanced mitochondrial respiration and improved cellular bioenergetics within muscle tissue. -

 Sermorelin is a synthetic analogue of growth hormone–releasing hormone (GHRH) that is commonly used to evaluate and enhance endogenous growth hormone production. Research interest in sermorelin stems from its potential roles in promoting bone density, minimizing scar formation, mitigating cognitive decline associated with dementia, and reducing seizure occurrence.
Sermorelin is a synthetic analogue of growth hormone–releasing hormone (GHRH) that is commonly used to evaluate and enhance endogenous growth hormone production. Research interest in sermorelin stems from its potential roles in promoting bone density, minimizing scar formation, mitigating cognitive decline associated with dementia, and reducing seizure occurrence. -

 PT-141, also known by its clinical name Bremelanotide, is a synthetic peptide derived from alpha-melanocyte-stimulating hormone (α-MSH) through extensive structural modifications. It has undergone clinical evaluation for the treatment of hypoactive sexual desire disorder (HSDD) in both men and women, as well as for applications in acute hemorrhagic conditions. PT-141 functions as an agonist at melanocortin-4 (MC4R) and melanocortin-1 (MC1R) receptors. Evidence from research indicates that it enhances sexual arousal and exerts immunomodulatory effects.
PT-141, also known by its clinical name Bremelanotide, is a synthetic peptide derived from alpha-melanocyte-stimulating hormone (α-MSH) through extensive structural modifications. It has undergone clinical evaluation for the treatment of hypoactive sexual desire disorder (HSDD) in both men and women, as well as for applications in acute hemorrhagic conditions. PT-141 functions as an agonist at melanocortin-4 (MC4R) and melanocortin-1 (MC1R) receptors. Evidence from research indicates that it enhances sexual arousal and exerts immunomodulatory effects. -

 Oxytocin is a peptide hormone with key physiological roles in sexual reproduction, childbirth, maternal-infant bonding during lactation, and tissue repair. Beyond these established functions, emerging evidence indicates that oxytocin may enhance cognitive performance, reduce cardiovascular risk, and mitigate complications associated with diabetes.
Oxytocin is a peptide hormone with key physiological roles in sexual reproduction, childbirth, maternal-infant bonding during lactation, and tissue repair. Beyond these established functions, emerging evidence indicates that oxytocin may enhance cognitive performance, reduce cardiovascular risk, and mitigate complications associated with diabetes. -

 Nicotinamide adenine dinucleotide (NAD+) is an essential pyridine nucleotide and ubiquitous coenzyme present in all living cells, where it plays a central role in cellular metabolism and bioenergetics. Acting as a redox mediator, NAD+ undergoes continuous interconversion between its oxidized (NAD+) and reduced (NADH) states, thereby facilitating electron transfer reactions fundamental to oxidative phosphorylation and ATP production. Beyond its canonical role in energy metabolism, NAD+ serves as a critical co-substrate in more than 500 enzymatic reactions, underscoring its broad involvement in cellular homeostasis. Accumulating evidence suggests that maintenance of NAD+ levels contributes to enhanced skeletal muscle function, neuroprotection, and attenuation of age-associated physiological decline.
Nicotinamide adenine dinucleotide (NAD+) is an essential pyridine nucleotide and ubiquitous coenzyme present in all living cells, where it plays a central role in cellular metabolism and bioenergetics. Acting as a redox mediator, NAD+ undergoes continuous interconversion between its oxidized (NAD+) and reduced (NADH) states, thereby facilitating electron transfer reactions fundamental to oxidative phosphorylation and ATP production. Beyond its canonical role in energy metabolism, NAD+ serves as a critical co-substrate in more than 500 enzymatic reactions, underscoring its broad involvement in cellular homeostasis. Accumulating evidence suggests that maintenance of NAD+ levels contributes to enhanced skeletal muscle function, neuroprotection, and attenuation of age-associated physiological decline. -

 Nicotinamide adenine dinucleotide (NAD+) is an essential pyridine nucleotide and ubiquitous coenzyme present in all living cells, where it plays a central role in cellular metabolism and bioenergetics. Acting as a redox mediator, NAD+ undergoes continuous interconversion between its oxidized (NAD+) and reduced (NADH) states, thereby facilitating electron transfer reactions fundamental to oxidative phosphorylation and ATP production. Beyond its canonical role in energy metabolism, NAD+ serves as a critical co-substrate in more than 500 enzymatic reactions, underscoring its broad involvement in cellular homeostasis. Accumulating evidence suggests that maintenance of NAD+ levels contributes to enhanced skeletal muscle function, neuroprotection, and attenuation of age-associated physiological decline.
Nicotinamide adenine dinucleotide (NAD+) is an essential pyridine nucleotide and ubiquitous coenzyme present in all living cells, where it plays a central role in cellular metabolism and bioenergetics. Acting as a redox mediator, NAD+ undergoes continuous interconversion between its oxidized (NAD+) and reduced (NADH) states, thereby facilitating electron transfer reactions fundamental to oxidative phosphorylation and ATP production. Beyond its canonical role in energy metabolism, NAD+ serves as a critical co-substrate in more than 500 enzymatic reactions, underscoring its broad involvement in cellular homeostasis. Accumulating evidence suggests that maintenance of NAD+ levels contributes to enhanced skeletal muscle function, neuroprotection, and attenuation of age-associated physiological decline. -
 Melanotan-2 (MT-2) is a synthetic analogue of alpha-melanocyte-stimulating hormone (α-MSH), originally developed in the 1980s. Research indicates that MT-2 may influence several physiological processes, including enhancement of sexual arousal, reduction of compulsive or addictive behaviors, appetite suppression, and stimulation of melanin synthesis. By activating melanocytes, MT-2 promotes increased skin pigmentation. Preliminary studies have also suggested a potential role in supporting neurodevelopmental outcomes, with some evidence pointing to possible benefits in early interventions for autism.
Melanotan-2 (MT-2) is a synthetic analogue of alpha-melanocyte-stimulating hormone (α-MSH), originally developed in the 1980s. Research indicates that MT-2 may influence several physiological processes, including enhancement of sexual arousal, reduction of compulsive or addictive behaviors, appetite suppression, and stimulation of melanin synthesis. By activating melanocytes, MT-2 promotes increased skin pigmentation. Preliminary studies have also suggested a potential role in supporting neurodevelopmental outcomes, with some evidence pointing to possible benefits in early interventions for autism. -
 Melanotan 1 is a synthetic analogue closely related to the naturally occurring peptide α-melanocyte-stimulating hormone (α-MSH, also referred to as Melanotan 2). α-MSH plays a key role in regulating skin and hair pigmentation by acting on melanocytes through strong binding to the melanocortin-1 receptor (MC1R). As a non-selective full agonist, α-MSH also activates melanocortin receptors 1, 3, 4, and 5. Melanotan 1 differs from α-MSH by a single amino acid substitution and was originally developed as a sunless tanning agent. Early studies confirmed its ability to induce pigmentation but also revealed broader effects, including changes in baseline metabolism. Research on Melanotan 1 and related melanocortin-binding peptides has since provided valuable insights into the melanocortin signaling system.
Melanotan 1 is a synthetic analogue closely related to the naturally occurring peptide α-melanocyte-stimulating hormone (α-MSH, also referred to as Melanotan 2). α-MSH plays a key role in regulating skin and hair pigmentation by acting on melanocytes through strong binding to the melanocortin-1 receptor (MC1R). As a non-selective full agonist, α-MSH also activates melanocortin receptors 1, 3, 4, and 5. Melanotan 1 differs from α-MSH by a single amino acid substitution and was originally developed as a sunless tanning agent. Early studies confirmed its ability to induce pigmentation but also revealed broader effects, including changes in baseline metabolism. Research on Melanotan 1 and related melanocortin-binding peptides has since provided valuable insights into the melanocortin signaling system. -

 MOTS-c is a 16–amino acid mitochondrial-derived peptide (MDP) that plays a key role in metabolic regulation. Produced within mitochondria, it helps maintain cellular energy balance by influencing glucose metabolism, insulin sensitivity, and stress-response pathways. MOTS-c can translocate to the nucleus, where it regulates the expression of genes involved in mitochondrial biogenesis and metabolic adaptation, particularly during metabolic stress. Studies indicate that MOTS-c enhances exercise performance, reduces obesity and insulin resistance, and provides protective effects against conditions such as osteoporosis, metabolic disorders, and age-related diseases. In summary, MOTS-c supports metabolic homeostasis, healthy aging, and improved physical performance while mitigating the risk of obesity, insulin resistance, and related disease states.
MOTS-c is a 16–amino acid mitochondrial-derived peptide (MDP) that plays a key role in metabolic regulation. Produced within mitochondria, it helps maintain cellular energy balance by influencing glucose metabolism, insulin sensitivity, and stress-response pathways. MOTS-c can translocate to the nucleus, where it regulates the expression of genes involved in mitochondrial biogenesis and metabolic adaptation, particularly during metabolic stress. Studies indicate that MOTS-c enhances exercise performance, reduces obesity and insulin resistance, and provides protective effects against conditions such as osteoporosis, metabolic disorders, and age-related diseases. In summary, MOTS-c supports metabolic homeostasis, healthy aging, and improved physical performance while mitigating the risk of obesity, insulin resistance, and related disease states. -

 MOTS-c is a 16–amino acid mitochondrial-derived peptide (MDP) that plays a key role in metabolic regulation. Produced within mitochondria, it helps maintain cellular energy balance by influencing glucose metabolism, insulin sensitivity, and stress-response pathways. MOTS-c can translocate to the nucleus, where it regulates the expression of genes involved in mitochondrial biogenesis and metabolic adaptation, particularly during metabolic stress. Studies indicate that MOTS-c enhances exercise performance, reduces obesity and insulin resistance, and provides protective effects against conditions such as osteoporosis, metabolic disorders, and age-related diseases. In summary, MOTS-c supports metabolic homeostasis, healthy aging, and improved physical performance while mitigating the risk of obesity, insulin resistance, and related disease states.
MOTS-c is a 16–amino acid mitochondrial-derived peptide (MDP) that plays a key role in metabolic regulation. Produced within mitochondria, it helps maintain cellular energy balance by influencing glucose metabolism, insulin sensitivity, and stress-response pathways. MOTS-c can translocate to the nucleus, where it regulates the expression of genes involved in mitochondrial biogenesis and metabolic adaptation, particularly during metabolic stress. Studies indicate that MOTS-c enhances exercise performance, reduces obesity and insulin resistance, and provides protective effects against conditions such as osteoporosis, metabolic disorders, and age-related diseases. In summary, MOTS-c supports metabolic homeostasis, healthy aging, and improved physical performance while mitigating the risk of obesity, insulin resistance, and related disease states. -
 KPV is a synthetic tripeptide (a short chain of three amino acids: lysine-proline-valine) that is derived from the larger hormone alpha-melanocyte stimulating hormone (α-MSH). It is a small peptide drug candidate with strong anti-inflammatory and protective properties, derived from the larger α-MSH hormone, and is being investigated as a targeted therapy for inflammatory disorders.
KPV is a synthetic tripeptide (a short chain of three amino acids: lysine-proline-valine) that is derived from the larger hormone alpha-melanocyte stimulating hormone (α-MSH). It is a small peptide drug candidate with strong anti-inflammatory and protective properties, derived from the larger α-MSH hormone, and is being investigated as a targeted therapy for inflammatory disorders. -
 Ipamorelin is a synthetic pentapeptide originally developed by Novo Nordisk as part of their exploration of selective growth hormone secretagogues. Structurally, it is a short chain of five amino acids, designed to act as an agonist at the ghrelin or growth hormone secretagogue receptor (GHS-R1a). By binding to this receptor in the hypothalamus and pituitary gland, ipamorelin mimics the activity of the natural “hunger hormone” ghrelin, stimulating the pituitary to release growth hormone (GH) in a pulsatile and physiologically natural rhythm. This mechanism contrasts with direct growth hormone injections, which deliver GH in a non-pulsatile, supra-physiological manner.
Ipamorelin is a synthetic pentapeptide originally developed by Novo Nordisk as part of their exploration of selective growth hormone secretagogues. Structurally, it is a short chain of five amino acids, designed to act as an agonist at the ghrelin or growth hormone secretagogue receptor (GHS-R1a). By binding to this receptor in the hypothalamus and pituitary gland, ipamorelin mimics the activity of the natural “hunger hormone” ghrelin, stimulating the pituitary to release growth hormone (GH) in a pulsatile and physiologically natural rhythm. This mechanism contrasts with direct growth hormone injections, which deliver GH in a non-pulsatile, supra-physiological manner. -
 IGF-1 LR3 (Insulin-like Growth Factor-1 Long R3) is a synthetic, engineered variant of insulin-like growth factor-1 designed for enhanced stability and activity. Unlike native IGF-1, IGF-1 LR3 has a reduced affinity for IGF-binding proteins, allowing it to remain biologically active for up to 120 times longer. This extended half-life increases its physiological impact, promoting enhanced cell proliferation, fat metabolism, and muscle growth through the suppression of myostatin. Additionally, emerging studies indicate potential applications in supporting lactation in postpartum mothers.
IGF-1 LR3 (Insulin-like Growth Factor-1 Long R3) is a synthetic, engineered variant of insulin-like growth factor-1 designed for enhanced stability and activity. Unlike native IGF-1, IGF-1 LR3 has a reduced affinity for IGF-binding proteins, allowing it to remain biologically active for up to 120 times longer. This extended half-life increases its physiological impact, promoting enhanced cell proliferation, fat metabolism, and muscle growth through the suppression of myostatin. Additionally, emerging studies indicate potential applications in supporting lactation in postpartum mothers. -
 Tirzepatide is a dual agonist targeting the glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors—novel dual incretin-based therapy. Its design integrates both GLP-1 and GIP actions in a single molecule, enhancing insulin secretion, reducing glucagon levels, delaying gastric emptying, and promoting satiety through central nervous system pathways.
Tirzepatide is a dual agonist targeting the glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors—novel dual incretin-based therapy. Its design integrates both GLP-1 and GIP actions in a single molecule, enhancing insulin secretion, reducing glucagon levels, delaying gastric emptying, and promoting satiety through central nervous system pathways. -
 Tirzepatide is a dual agonist targeting the glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors—novel dual incretin-based therapy. Its design integrates both GLP-1 and GIP actions in a single molecule, enhancing insulin secretion, reducing glucagon levels, delaying gastric emptying, and promoting satiety through central nervous system pathways.
Tirzepatide is a dual agonist targeting the glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors—novel dual incretin-based therapy. Its design integrates both GLP-1 and GIP actions in a single molecule, enhancing insulin secretion, reducing glucagon levels, delaying gastric emptying, and promoting satiety through central nervous system pathways. -
 Tirzepatide is a dual agonist targeting the glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors—novel dual incretin-based therapy. Its design integrates both GLP-1 and GIP actions in a single molecule, enhancing insulin secretion, reducing glucagon levels, delaying gastric emptying, and promoting satiety through central nervous system pathways.
Tirzepatide is a dual agonist targeting the glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors—novel dual incretin-based therapy. Its design integrates both GLP-1 and GIP actions in a single molecule, enhancing insulin secretion, reducing glucagon levels, delaying gastric emptying, and promoting satiety through central nervous system pathways. -
 Tirzepatide is a dual agonist targeting the glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors—novel dual incretin-based therapy. Its design integrates both GLP-1 and GIP actions in a single molecule, enhancing insulin secretion, reducing glucagon levels, delaying gastric emptying, and promoting satiety through central nervous system pathways.
Tirzepatide is a dual agonist targeting the glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors—novel dual incretin-based therapy. Its design integrates both GLP-1 and GIP actions in a single molecule, enhancing insulin secretion, reducing glucagon levels, delaying gastric emptying, and promoting satiety through central nervous system pathways. -

 Tirzepatide is a dual agonist targeting the glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors—novel dual incretin-based therapy. Its design integrates both GLP-1 and GIP actions in a single molecule, enhancing insulin secretion, reducing glucagon levels, delaying gastric emptying, and promoting satiety through central nervous system pathways.
Tirzepatide is a dual agonist targeting the glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors—novel dual incretin-based therapy. Its design integrates both GLP-1 and GIP actions in a single molecule, enhancing insulin secretion, reducing glucagon levels, delaying gastric emptying, and promoting satiety through central nervous system pathways. -
 Semaglutide is a long-acting glucagon-like peptide-1 receptor agonist (GLP-1 RA) developed for the treatment of type 2 diabetes and obesity. As an analogue of endogenous GLP-1, semaglutide binds to GLP-1 receptors to stimulate glucose-dependent insulin secretion, inhibit glucagon release, delay gastric emptying, and promote satiety, thereby improving glycemic control and reducing body weight (Nauck & Meier, 2019). Its molecular modifications, including substitution of alanine at position 8 and attachment of a C18 fatty diacid chain, extend its half-life to approximately one week, enabling once-weekly dosing (Marso et al., 2016). Semaglutide was initially approved for the management of type 2 diabetes under the trade name Ozempic and later for chronic weight management under the brand Wegovy. Large-scale clinical trials, such as the SUSTAIN and STEP programs, demonstrated significant reductions in glycated hemoglobin (HbA1c), meaningful weight loss, and favorable cardiovascular outcomes in patients at high risk (Wilding et al., 2021; Marso et al., 2016). Beyond diabetes and obesity, ongoing research is evaluating semaglutide’s potential role in non-alcoholic steatohepatitis (NASH), cardiovascular disease, and neurodegenerative disorders (Newsome et al., 2021). Given its robust clinical efficacy and broad therapeutic potential, semaglutide represents a major advancement in metabolic medicine and has reshaped the treatment paradigm for both diabetes and obesity.
Semaglutide is a long-acting glucagon-like peptide-1 receptor agonist (GLP-1 RA) developed for the treatment of type 2 diabetes and obesity. As an analogue of endogenous GLP-1, semaglutide binds to GLP-1 receptors to stimulate glucose-dependent insulin secretion, inhibit glucagon release, delay gastric emptying, and promote satiety, thereby improving glycemic control and reducing body weight (Nauck & Meier, 2019). Its molecular modifications, including substitution of alanine at position 8 and attachment of a C18 fatty diacid chain, extend its half-life to approximately one week, enabling once-weekly dosing (Marso et al., 2016). Semaglutide was initially approved for the management of type 2 diabetes under the trade name Ozempic and later for chronic weight management under the brand Wegovy. Large-scale clinical trials, such as the SUSTAIN and STEP programs, demonstrated significant reductions in glycated hemoglobin (HbA1c), meaningful weight loss, and favorable cardiovascular outcomes in patients at high risk (Wilding et al., 2021; Marso et al., 2016). Beyond diabetes and obesity, ongoing research is evaluating semaglutide’s potential role in non-alcoholic steatohepatitis (NASH), cardiovascular disease, and neurodegenerative disorders (Newsome et al., 2021). Given its robust clinical efficacy and broad therapeutic potential, semaglutide represents a major advancement in metabolic medicine and has reshaped the treatment paradigm for both diabetes and obesity. -
 Semaglutide is a long-acting glucagon-like peptide-1 receptor agonist (GLP-1 RA) developed for the treatment of type 2 diabetes and obesity. As an analogue of endogenous GLP-1, semaglutide binds to GLP-1 receptors to stimulate glucose-dependent insulin secretion, inhibit glucagon release, delay gastric emptying, and promote satiety, thereby improving glycemic control and reducing body weight (Nauck & Meier, 2019). Its molecular modifications, including substitution of alanine at position 8 and attachment of a C18 fatty diacid chain, extend its half-life to approximately one week, enabling once-weekly dosing (Marso et al., 2016). Semaglutide was initially approved for the management of type 2 diabetes under the trade name Ozempic and later for chronic weight management under the brand Wegovy. Large-scale clinical trials, such as the SUSTAIN and STEP programs, demonstrated significant reductions in glycated hemoglobin (HbA1c), meaningful weight loss, and favorable cardiovascular outcomes in patients at high risk (Wilding et al., 2021; Marso et al., 2016). Beyond diabetes and obesity, ongoing research is evaluating semaglutide’s potential role in non-alcoholic steatohepatitis (NASH), cardiovascular disease, and neurodegenerative disorders (Newsome et al., 2021). Given its robust clinical efficacy and broad therapeutic potential, semaglutide represents a major advancement in metabolic medicine and has reshaped the treatment paradigm for both diabetes and obesity.
Semaglutide is a long-acting glucagon-like peptide-1 receptor agonist (GLP-1 RA) developed for the treatment of type 2 diabetes and obesity. As an analogue of endogenous GLP-1, semaglutide binds to GLP-1 receptors to stimulate glucose-dependent insulin secretion, inhibit glucagon release, delay gastric emptying, and promote satiety, thereby improving glycemic control and reducing body weight (Nauck & Meier, 2019). Its molecular modifications, including substitution of alanine at position 8 and attachment of a C18 fatty diacid chain, extend its half-life to approximately one week, enabling once-weekly dosing (Marso et al., 2016). Semaglutide was initially approved for the management of type 2 diabetes under the trade name Ozempic and later for chronic weight management under the brand Wegovy. Large-scale clinical trials, such as the SUSTAIN and STEP programs, demonstrated significant reductions in glycated hemoglobin (HbA1c), meaningful weight loss, and favorable cardiovascular outcomes in patients at high risk (Wilding et al., 2021; Marso et al., 2016). Beyond diabetes and obesity, ongoing research is evaluating semaglutide’s potential role in non-alcoholic steatohepatitis (NASH), cardiovascular disease, and neurodegenerative disorders (Newsome et al., 2021). Given its robust clinical efficacy and broad therapeutic potential, semaglutide represents a major advancement in metabolic medicine and has reshaped the treatment paradigm for both diabetes and obesity. -

 Semaglutide is a long-acting glucagon-like peptide-1 receptor agonist (GLP-1 RA) developed for the treatment of type 2 diabetes and obesity. As an analogue of endogenous GLP-1, semaglutide binds to GLP-1 receptors to stimulate glucose-dependent insulin secretion, inhibit glucagon release, delay gastric emptying, and promote satiety, thereby improving glycemic control and reducing body weight (Nauck & Meier, 2019). Its molecular modifications, including substitution of alanine at position 8 and attachment of a C18 fatty diacid chain, extend its half-life to approximately one week, enabling once-weekly dosing (Marso et al., 2016). Semaglutide was initially approved for the management of type 2 diabetes under the trade name Ozempic and later for chronic weight management under the brand Wegovy. Large-scale clinical trials, such as the SUSTAIN and STEP programs, demonstrated significant reductions in glycated hemoglobin (HbA1c), meaningful weight loss, and favorable cardiovascular outcomes in patients at high risk (Wilding et al., 2021; Marso et al., 2016). Beyond diabetes and obesity, ongoing research is evaluating semaglutide’s potential role in non-alcoholic steatohepatitis (NASH), cardiovascular disease, and neurodegenerative disorders (Newsome et al., 2021). Given its robust clinical efficacy and broad therapeutic potential, semaglutide represents a major advancement in metabolic medicine and has reshaped the treatment paradigm for both diabetes and obesity.
Semaglutide is a long-acting glucagon-like peptide-1 receptor agonist (GLP-1 RA) developed for the treatment of type 2 diabetes and obesity. As an analogue of endogenous GLP-1, semaglutide binds to GLP-1 receptors to stimulate glucose-dependent insulin secretion, inhibit glucagon release, delay gastric emptying, and promote satiety, thereby improving glycemic control and reducing body weight (Nauck & Meier, 2019). Its molecular modifications, including substitution of alanine at position 8 and attachment of a C18 fatty diacid chain, extend its half-life to approximately one week, enabling once-weekly dosing (Marso et al., 2016). Semaglutide was initially approved for the management of type 2 diabetes under the trade name Ozempic and later for chronic weight management under the brand Wegovy. Large-scale clinical trials, such as the SUSTAIN and STEP programs, demonstrated significant reductions in glycated hemoglobin (HbA1c), meaningful weight loss, and favorable cardiovascular outcomes in patients at high risk (Wilding et al., 2021; Marso et al., 2016). Beyond diabetes and obesity, ongoing research is evaluating semaglutide’s potential role in non-alcoholic steatohepatitis (NASH), cardiovascular disease, and neurodegenerative disorders (Newsome et al., 2021). Given its robust clinical efficacy and broad therapeutic potential, semaglutide represents a major advancement in metabolic medicine and has reshaped the treatment paradigm for both diabetes and obesity. -

 GHK-Cu is an endogenous peptide naturally present in human plasma, urine, and saliva. Preclinical studies in animal models indicate that GHK-Cu enhances wound repair, supports immune function, and promotes skin regeneration by stimulating collagen synthesis, activating fibroblasts, and facilitating angiogenesis. Evidence also suggests that it functions as a feedback signal released following tissue injury. Additionally, GHK-Cu exhibits antioxidant properties by mitigating free radical–induced damage.
GHK-Cu is an endogenous peptide naturally present in human plasma, urine, and saliva. Preclinical studies in animal models indicate that GHK-Cu enhances wound repair, supports immune function, and promotes skin regeneration by stimulating collagen synthesis, activating fibroblasts, and facilitating angiogenesis. Evidence also suggests that it functions as a feedback signal released following tissue injury. Additionally, GHK-Cu exhibits antioxidant properties by mitigating free radical–induced damage. -

 Epithalon (Epitalon) is a synthetic tetrapeptide (Ala-Glu-Asp-Gly) originally developed as an analogue of the naturally occurring pineal peptide complex, Epithalamin. It has been proposed to act as a modulator of telomerase activity, the ribonucleoprotein enzyme complex responsible for the preservation and elongation of telomeric DNA sequences at chromosomal termini. Experimental evidence suggests that Epithalon may facilitate telomere extension, thereby enhancing genomic stability, delaying replicative senescence, and exerting potential geroprotective effects through the attenuation of age-associated cellular decline.
Epithalon (Epitalon) is a synthetic tetrapeptide (Ala-Glu-Asp-Gly) originally developed as an analogue of the naturally occurring pineal peptide complex, Epithalamin. It has been proposed to act as a modulator of telomerase activity, the ribonucleoprotein enzyme complex responsible for the preservation and elongation of telomeric DNA sequences at chromosomal termini. Experimental evidence suggests that Epithalon may facilitate telomere extension, thereby enhancing genomic stability, delaying replicative senescence, and exerting potential geroprotective effects through the attenuation of age-associated cellular decline. -

 Epithalon (Epitalon) is a synthetic tetrapeptide (Ala-Glu-Asp-Gly) originally developed as an analogue of the naturally occurring pineal peptide complex, Epithalamin. It has been proposed to act as a modulator of telomerase activity, the ribonucleoprotein enzyme complex responsible for the preservation and elongation of telomeric DNA sequences at chromosomal termini. Experimental evidence suggests that Epithalon may facilitate telomere extension, thereby enhancing genomic stability, delaying replicative senescence, and exerting potential geroprotective effects through the attenuation of age-associated cellular decline.
Epithalon (Epitalon) is a synthetic tetrapeptide (Ala-Glu-Asp-Gly) originally developed as an analogue of the naturally occurring pineal peptide complex, Epithalamin. It has been proposed to act as a modulator of telomerase activity, the ribonucleoprotein enzyme complex responsible for the preservation and elongation of telomeric DNA sequences at chromosomal termini. Experimental evidence suggests that Epithalon may facilitate telomere extension, thereby enhancing genomic stability, delaying replicative senescence, and exerting potential geroprotective effects through the attenuation of age-associated cellular decline. -
 Epithalon (Epitalon) is a synthetic tetrapeptide (Ala-Glu-Asp-Gly) originally developed as an analogue of the naturally occurring pineal peptide complex, Epithalamin. It has been proposed to act as a modulator of telomerase activity, the ribonucleoprotein enzyme complex responsible for the preservation and elongation of telomeric DNA sequences at chromosomal termini. Experimental evidence suggests that Epithalon may facilitate telomere extension, thereby enhancing genomic stability, delaying replicative senescence, and exerting potential geroprotective effects through the attenuation of age-associated cellular decline.
Epithalon (Epitalon) is a synthetic tetrapeptide (Ala-Glu-Asp-Gly) originally developed as an analogue of the naturally occurring pineal peptide complex, Epithalamin. It has been proposed to act as a modulator of telomerase activity, the ribonucleoprotein enzyme complex responsible for the preservation and elongation of telomeric DNA sequences at chromosomal termini. Experimental evidence suggests that Epithalon may facilitate telomere extension, thereby enhancing genomic stability, delaying replicative senescence, and exerting potential geroprotective effects through the attenuation of age-associated cellular decline. -

 CJC-1295 No DAC (Modified GRF) is a shortened peptide derivative of growth hormone–releasing hormone (GHRH). Originally developed in the 1980s, studies on modGRF suggest it may support muscle repair and development, speed up wound recovery, enhance bone strength, boost fat metabolism, and promote overall metabolic health. Research also indicates it could play a role in regulating blood sugar levels and supporting immune function.
CJC-1295 No DAC (Modified GRF) is a shortened peptide derivative of growth hormone–releasing hormone (GHRH). Originally developed in the 1980s, studies on modGRF suggest it may support muscle repair and development, speed up wound recovery, enhance bone strength, boost fat metabolism, and promote overall metabolic health. Research also indicates it could play a role in regulating blood sugar levels and supporting immune function. -

 CJC-1295 DAC is a synthetic peptide designed as an analog of growth hormone–releasing hormone (GHRH). Its primary purpose is to stimulate the pituitary gland to release more growth hormone, which subsequently elevates levels of IGF-1 (insulin-like growth factor 1). What sets the DAC version apart from the non-DAC form is the addition of a “Drug Affinity Complex.” This chemical modification allows CJC-1295 DAC to bind to albumin in the bloodstream, extending its half-life from just minutes to several days. Because of this extended duration, a single administration can keep growth hormone levels elevated for nearly a week, making it significantly more efficient in terms of dosing frequency compared to its short-lived counterpart. In research contexts, CJC-1295 DAC has been studied for its potential to promote tissue repair, support lean muscle growth, improve fat metabolism, enhance sleep quality, and contribute to overall cellular rejuvenation. These effects are largely connected to the consistent elevation of growth hormone and IGF-1, which play central roles in recovery, metabolism, and anti-aging pathways. Despite these promising avenues, CJC-1295 DAC remains a research chemical rather than an approved therapeutic drug. Human studies are limited, and while side effects observed have included injection site reactions, water retention, flushing, and tingling, the long-term implications are not yet fully understood. Because of this, its use outside of supervised research carries risks, and it is not approved by regulatory authorities for medical treatment.
CJC-1295 DAC is a synthetic peptide designed as an analog of growth hormone–releasing hormone (GHRH). Its primary purpose is to stimulate the pituitary gland to release more growth hormone, which subsequently elevates levels of IGF-1 (insulin-like growth factor 1). What sets the DAC version apart from the non-DAC form is the addition of a “Drug Affinity Complex.” This chemical modification allows CJC-1295 DAC to bind to albumin in the bloodstream, extending its half-life from just minutes to several days. Because of this extended duration, a single administration can keep growth hormone levels elevated for nearly a week, making it significantly more efficient in terms of dosing frequency compared to its short-lived counterpart. In research contexts, CJC-1295 DAC has been studied for its potential to promote tissue repair, support lean muscle growth, improve fat metabolism, enhance sleep quality, and contribute to overall cellular rejuvenation. These effects are largely connected to the consistent elevation of growth hormone and IGF-1, which play central roles in recovery, metabolism, and anti-aging pathways. Despite these promising avenues, CJC-1295 DAC remains a research chemical rather than an approved therapeutic drug. Human studies are limited, and while side effects observed have included injection site reactions, water retention, flushing, and tingling, the long-term implications are not yet fully understood. Because of this, its use outside of supervised research carries risks, and it is not approved by regulatory authorities for medical treatment.
Need help? Call Or Text us, and a team member will be happy to assist you. +1 (570) 539-9711
Need help? Call Or Text us, and a team member will be happy to assist you.
+1 (570) 539-9711